Cell testing with Liqcreate Bio-Med Clear - a biocompatible 3D-printing resin
Liqcreate collaborated with the biology department of the University of Applied Science, Eindhoven, The Netherlands on cell testing on resin 3D-printed parts. This research focused on the effect of 3D-printing resin, in this case Liqcreate Bio-Med Clear, on the adhesion, growth, cell division and survival of different cell cultures and bacteria in a controlled laboratory environment. Specifically, it is investigated how the biocompatible 3D-printing resin influenced cell adhesion, whether it promotes or inhibits the growth of cells and bacteria, and whether there are changes in cell death or bacterial survival rate. By mapping these interactions, the researchers not only want to contribute to the research into biocompatible 3D-printed materials, but also ensure the safety and effectiveness for potential medical and biological applications of these materials.

Image: Succesful adhesion and growth of MDA-MB-175-VII cells to a 3D-printed disc made with Liqcreate Bio-Med Clear resin. These are human epithelial-like cells isolated from a pleural effusion of person with a breast tumor.
Summary
This report examines the effect of Liqcreate ‘s Bio-Med Clear 3D-printing resin on cell cultures and bacteria in a controlled laboratory environment. The purpose of the study is to test the biological compatibility of the resin and to gain insight into the effect of the Bio-Med Clear resin on the adhesion, growth, cell division and survival of cells and bacteria.
This project was divided into two parts. In the first part, the effects of Bio-Med Clear resin on bacterial cells were tested. To test the above parameters, a bacterial suspension of Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli and Salmonella typhi was made in BHI medium, which was then incubated for 5 days in a 24-well plate containing discs 3D-printed with Liqcreate Bio-Med Clear resin. After incubation, the discs were washed with sterile physiological saline. The bacteria attached to the disc were analyzed using a spread plate on PCA agar.
In the second part, the impact of Bio-Med Clear with human cells was investigated. For this purpose, the MDA-MB-175-VII cell line, a human epithelial carcinoma cell line, was used. A cell suspension containing RPMI medium was added to a 24-well plate with Bio-Med Clear discs for 5 days, and the adhesion of the cells to the material was investigated with a ZOE Fluorescent Cell Imager.
From the results obtained, it can be concluded that the 3D-printed Bio-Med Clear material supports adhesion of both the tested bacteria and human cells. Additionally there was no significant different in cell growth and size during the incubation time. This conclusion indicates that the material could potentially be used for various purposes, such as prosthetics and laboratory applications such as well plates. To further explore the potential of the material, it is recommended to repeat the experiments, test other bacterial strains and cell types, increase the incubation time and examine larger areas of the material. These are factors that can still be investigated to ensure that the results found are representative.
From the results obtained, it can be concluded that the 3D-printed Bio-Med Clear material supports adhesion of both the tested bacteria and human cells. Additionally there was no significant different in cell growth and size during the incubation time.
Researchers from the University of Applied Science, Eindhoven, Netherlands
Testing Bacterial Cells on Bio-Med Clear
For performing the bacterial tests, two batches of BHI medium from Thermo Fisher Diagnostics were prepared per bacterial test. A 50 ml falcon centrifuge tube was filled with 40 ml of sterile BHI medium, and 100 ml of sterile BHI medium was placed in a glass sealable bottle. The BHI in the falcon centrifuge tube was used to make a bacterial suspension of one of the bacteria to be tested per experiment: Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli or Salmonella typhi. The bacterial suspensions were made by transferring one full sterile loopful with pure bacterial colony to the 40 ml sterile BHI. The contents of the 50 ml centrifuge tube were mixed with a VWR® VV3 Vortex mixer at maximum power, after which the tube was set aside for use. After making the bacterial suspension, both the bacterial suspension and the bottle with sterile BHI medium were placed in the LAF cabinet.
The rest of the experiment was performed inside the LAF cabinet. A Greiner CELLSTAR® 24-well plate was removed from the packaging and the wells were numbered on the bottom with a permanent marker. This was done from right to left and from top to bottom, so that the order remained correct when the plate was turned upside down. Using tweezers, autoclaved Bio-Med Clear discs were placed in the first eighteen wells of the plate, one disc per well. Then, 1.6 ml of bacterial suspension of the bacterium to be tested was pipetted into the first twenty-one wells. In this way, three wells without discs were also filled with bacterial suspension. These wells served as positive controls. To prevent contamination, 1.60 mL of sterile BHI medium was pipetted into three wells per experiment in a separate 24-well plate as the negative control. This plate was opened each time a bacterial plate was inserted and closed until the next plate was inserted. In this way, the conditions of the inserted plates were the same, but contamination by, for example aerosol formation was prevented. Both 24-well plates were then incubated for 48 hours at 37°C.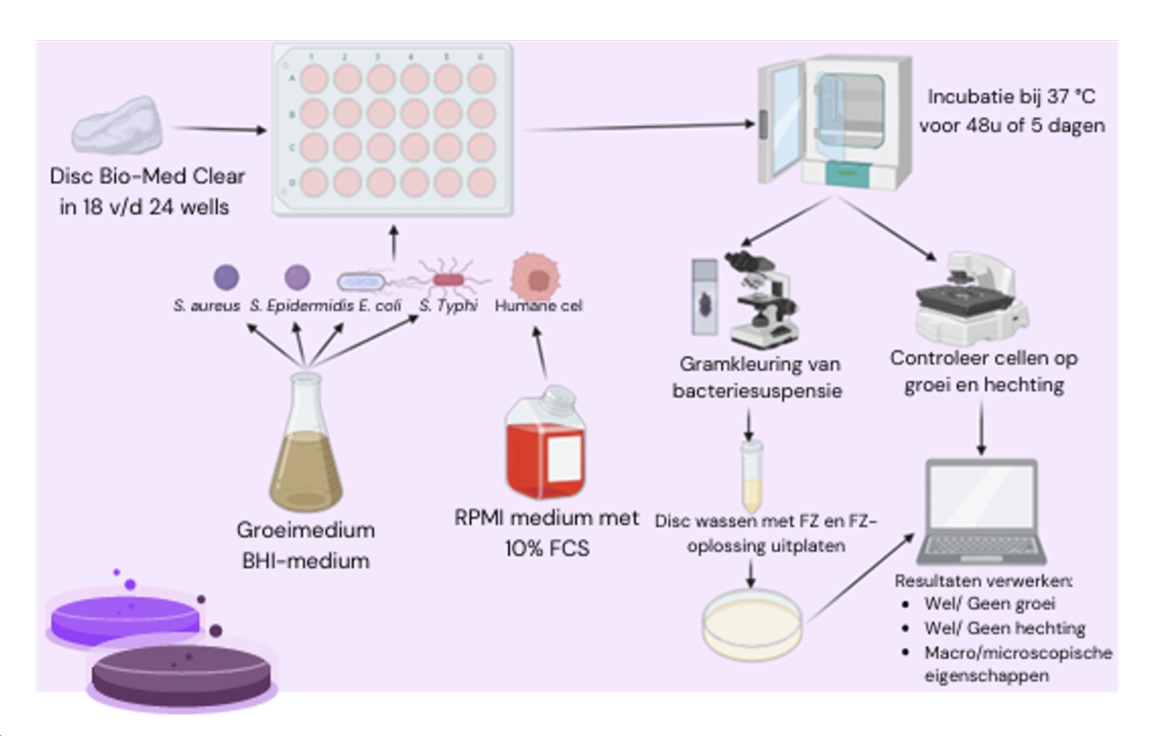
After incubation of the plates, the results of the test were generated. First, a Gram stain was made of the suspension from eight different wells of the 24-well plate. The Gram preparations were examined under a microscope to check for the presence of bacteria in the suspension and to identify the bacteria. After this, the bacterial suspension was pipetted away from the same 8 wells from which the Gram stain was performed earlier. The empty wells were washed with 1.6 ml of sterile physiological saline. For this, the plate was placed on the shaking incubator for five minutes, which rotated the plate at a speed of 200 RPM. In this way, any bacteria that were still present in the well but not attached to the disc were washed away. After washing, the discs were removed from the wells and transferred to a 5 mL falcon tube containing 2 mL of sterile physiological saline. These tubes were also placed on the shaking incubator for 30 minutes. So that any bacteria that may have attached to the disc could be washed off. After this, 100 μl was taken from each tube. This 100 μl was plated onto VWR® Plate Count Agar using the spread plate method and a triangle spatula. A spread plate was also made from one of the blank wells of the blank 24-well plate as a control. All spread plates were then incubated for 24 hours at 37°C.
After incubation, the PCA plates were used to determine whether the bacteria had attached to the Bio-Med Clear resin. This was assessed by looking at the colony growth on the PCA plates. Growth on the PCA plates indicated that bacteria had attached to the disc, and no growth meant that no bacteria had attached to the disc.
Testing MDA-MB-175-VII cells on Bio-Med Clear
This cell test was performed using a confluent T75 flask with cultured MDA-MB-175-VII cells. During the culture process, a cell count was performed on the cell suspension regularly, in order to keep a close eye on the vitality and the number of living cells.
To perform the test, the cells were first detached from the wall of the T75 flask by adding 3 mL of trypsin-EDTA 1x to the flask. The flask was then incubated in a 37°C incubator for five minutes, so that the trypsin could release the protein bonds. After five minutes, the flask was placed back in the LAF cabinet, after which the remaining cells were detached from the bottom using a scraper. To neutralize the effect of the trypsin, 30 ml of RPMI cell culture medium from VWR® with 10% Avantor® Fetal Bovine Serum, preheated to 37°C, was added. The cells and the medium were then mixed by pipetting the liquid up and down several times with a pipette. The entire contents of the T75 flask were then transferred to a 50 ml falcon centrifuge tube. In addition, a 15 ml falcon centrifuge tube was filled with 10 ml of sterile RPMI cell culture medium in the LAF cabinet. This tube was used as a negative control.
After preparing the cell suspension, a Greiner CELLSTAR® 24-well plate was removed from the packaging. The wells were numbered on the bottom with a permanent marker. This was done from right to left and from top to bottom, so that the order remained correct when the plate was turned over. Using tweezers, autoclaved Bio-Med Clear discs were placed in the first eighteen wells of the plate, 1 disc per well. Subsequently, 1.6 ml of cell suspension was pipetted into the first twenty-one wells. As a result, three wells without discs were also filled with bacterial suspension, these wells served as positive controls. In the last three wells, 1.6 ml of sterile RPMI cell culture medium was pipetted, these wells served as negative controls. The 24-well plate was then incubated for 120 hours at 37°C with 5% CO2.
To generate the results, the 24-well plate was assessed both microscopically and macroscopically daily. Macroscopically, mainly the color and turbidity of the cell culture medium were observed, and with the aid of the ZOE Fluorescent Cell-Imager, the various wells could also be assessed microscopically. After five days, eight discs were removed from the wells. These discs were then placed on a microscope slide, allowing the discs to be viewed under the microscope. Using the ZOE Fluorescent Cell-Imager and a red light filter, it was finally determined whether or not the cells were attached to the plastic.
Results from bacterial tests
During this experiment, it was examined whether bacterial strains Staphylococcus aureus, Escherichia coli, Salmonella typhi and Staphylococcus epidermidis have the ability to adhere to the resin of Liqcreate’s Bio-Med Clear. In addition, it was examined whether Bio-Med Clear has an influence on the growth and multiplication of the bacterial strains.
To investigate this, 4 experiments were performed in which discs of Bio-Med Clear resin were incubated in 24-well plates with S. aureus, E. coli, S. epidermidis, and S. typhi bacterial suspensions. After incubation, a Gram stain was made of eight wells from each plate, to check whether bacteria were present in the suspension and to determine the bacteria. Due to the large number of results, it was decided to only discuss the results of the spread plates. The results of the 24-well plates and Gram stains of the four experiments performed are further elaborated in appendices I to IV.
After performing the Gram stains, the wells and discs were washed with sterile physiological saline. The physiological saline was plated over PCA plates using the spread plate method, after which the PCA plates were incubated for 24 hours at 37°C. The results of the PCA spread plates after incubation are shown below in table 2.
Table 2: Photos of the PCA spread plates of bacterial tests S. aureus, E. coli, S. epidermidis and S. typhi with Bio-Med Clear resin after 24 hours of incubation.
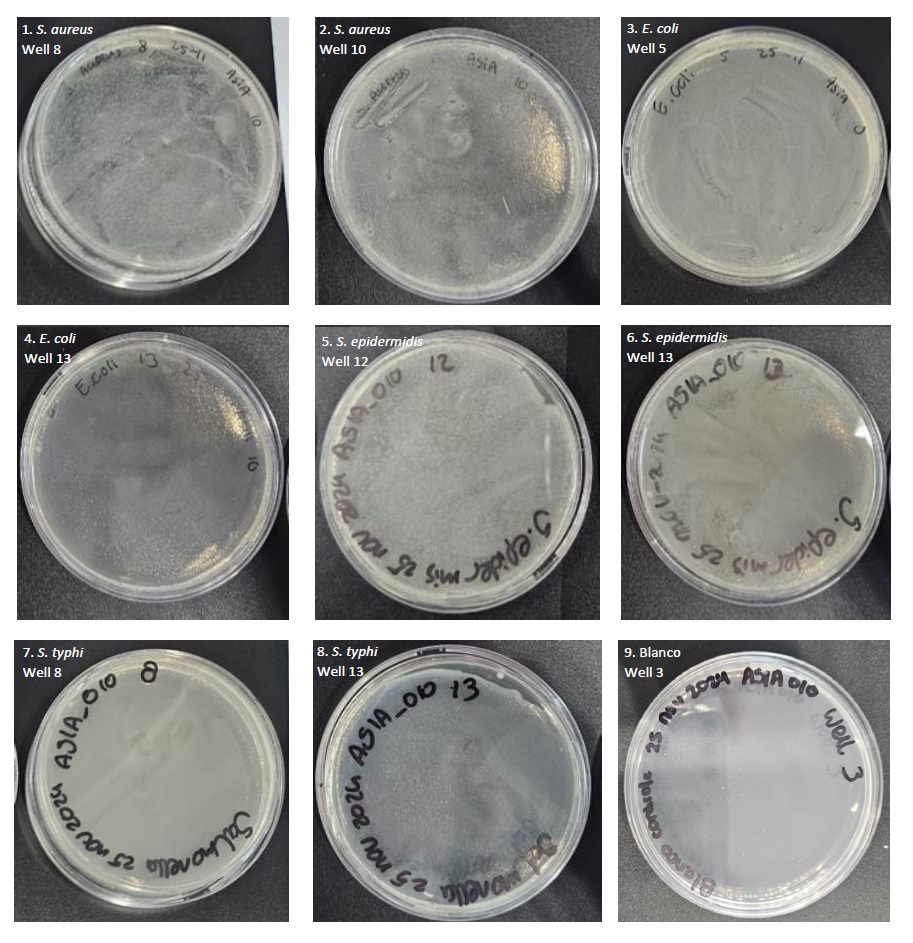
In table 2 above, the results of the four performed bacterial tests can be seen in the form of PCA spread plates. In photos 1 and 2, PCA spread plates are visible from the bacterial test with Staphylococcus aureus bacterial suspension, in photos 3 and 4, PCA spread plates are visible from the bacterial test with Escherichia coli bacterial suspension, in photos 5 and 6, PCA spread plates are visible from the bacterial test with Staphylococcus epidermidis bacterial suspension, and in photos 7 and 8, PCA spread plates are visible from the bacterial test with Salmonella typhi bacterial suspension.
Clear bacterial growth is visible on all plates. All plates show pure growth of the relevant bacterial species without signs of contamination or presence of the other bacterial species. In all four experiments, positive and negative controls were also included. The PCA spread plates of the positive controls showed pure growth of the relevant bacterial species, and here too there was no contamination or presence of the other bacterial species. A separate 24-well plate was taken for the negative control in order to prevent work contamination in this way. A clean streak was also made of these wells, which can be seen in photo 9 in table 2. Here it can be clearly seen that no bacterial growth occurred on the plate after incubation. This indicates that the entire process was carried out sterilely, and that no work contamination occurred anywhere.
Results from MDA-MB-175-VII cells
During this experiment it was examined whether MDA-MB-175-VII cells have the ability to adhere to the Bio-Med Clear resin. To investigate this, discs of Bio-Med Clear resin were incubated in a 24-well plate with a cell suspension consisting of MDA-MB-175-VII cells. The 24-well plate was then incubated for 120 hours at 37 °C and 5% CO2. To generate the results, the plate was checked daily both macroscopically and microscopically. Below in figure 2 a picture of the 24-well plate is visible after 120 hours of incubation.

The 24-well plate of the human cell test is laid out in the same way as the 24-well plates of the bacterial tests. The wells are numbered from 1 to 24, with the well at the top left corner being number 1. The numbering then continues downwards, making the well at the bottom left corner number 4. The numbering then continues from top to bottom again, making the second well in the top row number 5. This continues throughout the entire plate from top to bottom.
The first 18 wells contain both a disc and bacterial suspension. These wells can be recognized in the photo above by their pink/light orange color. Next, there are 3 wells with only cell suspension (well 19, 20 and 21). These wells can be recognized by their light pink color, and are used in this experiment as a positive control. The last 3 wells of the plate contain only sterile RPMI culture medium with 10% FCS, and are used in this experiment as a negative control.
The medium in the wells with a Bio-Med Clear disc still has a clear color after 120 hours of incubation, which indicates that no contamination has occurred in these wells. The medium in these wells does have a slightly different color compared to the positive control wells, but that is because the Bio-Med Clear resin disc has a yellow color of its own. As a result, the color of the liquid in the wells also appears to have a yellow/orange tint from above. From the side, the medium looked pink, and the color matched the color of the positive control wells. However, this was difficult to capture in the photo. The medium in the wells of the positive control also still has a clear colour after 120 hours of incubation, which indicates that no contamination has occurred in these wells. In the negative control wells, the medium in one of the three wells (well 22) has grown cloudy after approximately 4 days. It is unclear what the cause of this is, but it indicates that there is some contamination in the well, which means that this control may not be approved.
Since the contamination in the negative control only occurred in 1 of the 3 wells, it was decided after consultation with the experts to interpret the rest of the test results. In the other 2 wells of the negative control, there is no further indication of contamination, because the liquid in these wells is simply clear and has a nice dark pink colour. This probably means that there was a work contamination that occurred during the use of the test. After 120 hours, the fluid from the contaminated negative control well was pipetted so that the contamination could not spread further across the plate. The results of the test were then generated using the ZOE Fluorescent Cell-Imager.
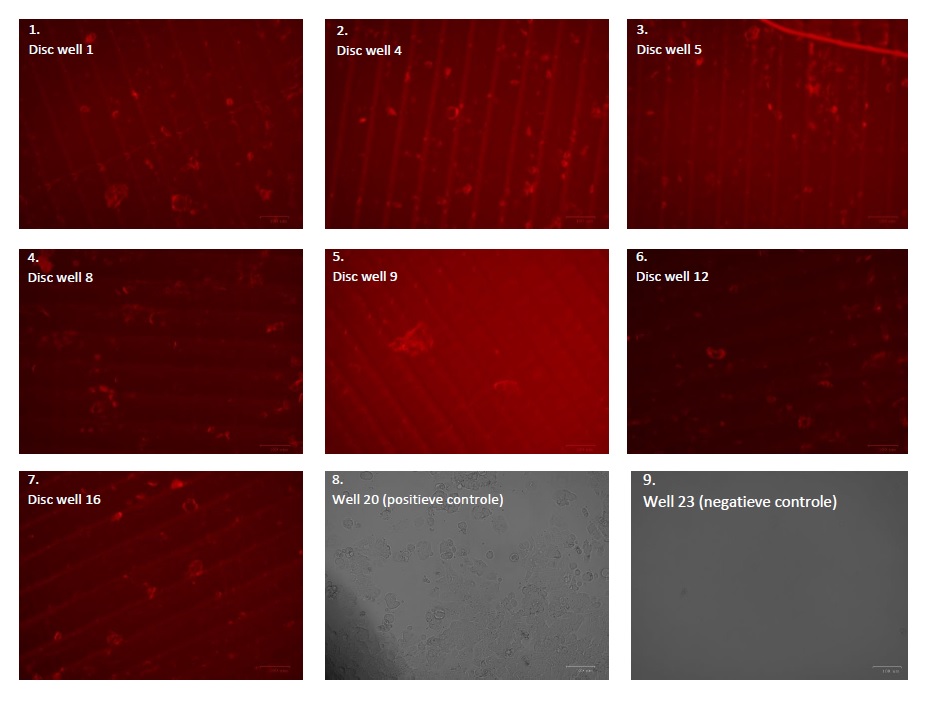
Table 3 above shows the results of testing the adhesion capacity of MDA-MB-175-VII cells to Bio-Med Clear discs. The discs were removed from the wells of the 24-well plate in Figure using tweezers and viewed on a microscope slide using the ZOE® Fluorescent Cell-Imager. For the plastic Bio-Med Clear discs, it was decided to view them with red fluorescence (see also photos 1 to 7 in Table 3), because this filter could generate the clearest results. The plastic is 3D printed, which means that it is built up in layers. The plastic can therefore be easily recognized in photos 1 to 7 by its ribbed structure.
All seven discs shown in Table 3 have spots on the plastic. This indicates that the cells have attached to the plastic during the incubation process and that the cells can grow around the plastic. In photo 8, an image of the positive control well is visible. This photo was taken with a normal light filter, and it is clearly visible that the cells in this image correspond to the cells/spots visible in photos 1 to 7 around the plastic Bio-Med Clear disc. In photo 9, an image of the negative control well is visible. No cells or other factors or contaminations are present in this medium. This indicates that the contamination from well 22 has not been transferred to the other negative control wells.
Because the positive and negative control wells did not contain a disc, it was possible to view the wells themselves directly under the microscope. This was not possible for the other wells, because it could not be determined whether the cells were attached to the plastic or to the bottom of the wells.
Conclusion
The aim of this study was to investigate the effect of Bio-Med Clear 3D printable plastic on the adhesion, growth, cell division and survival of various cell cultures and bacteria in a controlled laboratory environment. This study showed that both bacterial cells (S. aureus, S. epidermis, E. coli and S. typhi) and human cells (MDA-MB-175-VII) can adhere to the Bio-Med Clear resin. All experiments showed that the plastic does not further influence the growth and multiplication of the cells and bacteria, because after incubation with the plastic Bio-Med Clear resin discs, both bacterial growth and cell growth were similar compared to the positive control. This indicates that no harmful monomers are released from the plastic, which confirms that the plastic has been cured well enough and that the curing and washing process has been carried out efficiently by the company Liqcreate. Based on these findings, it can be concluded that Bio-Med Clear is suitable for applications where this biological compatibility is required.
Liqcreate Bio-Med Clear
Liqcreate Bio-Med Clear is a rigid clear biocompatble photopolymer resin and can be processed on most resin based 3D-printers. 3D-printed parts from this material exhibit biocompatible properties when post processed according to the processing instructions1. After washing and post-curing according to the instructions, printed parts from Liqcreate Bio-Med Clear pass the biocompatibility tests of:
| ○ Cytotoxicity | ISO 10993-5:2009 |
| ○ Sensitization | ISO 10993-10:2021 |
| ○ Irritation | ISO 10993-23:2021 |
Printed parts from Bio-Med Clear can be disinfected with commonly used disinfectants and sterilized by steam sterilization using an autoclave.
Key benefits |
3D-Printer compatibility |
| · Biocompatible | · Asiga series |
| · Steam sterilization possible | · Phrozen series |
| · High accuracy | · Elegoo & Anycubic series |
| · Dimensional stable | · And many more |
